Home
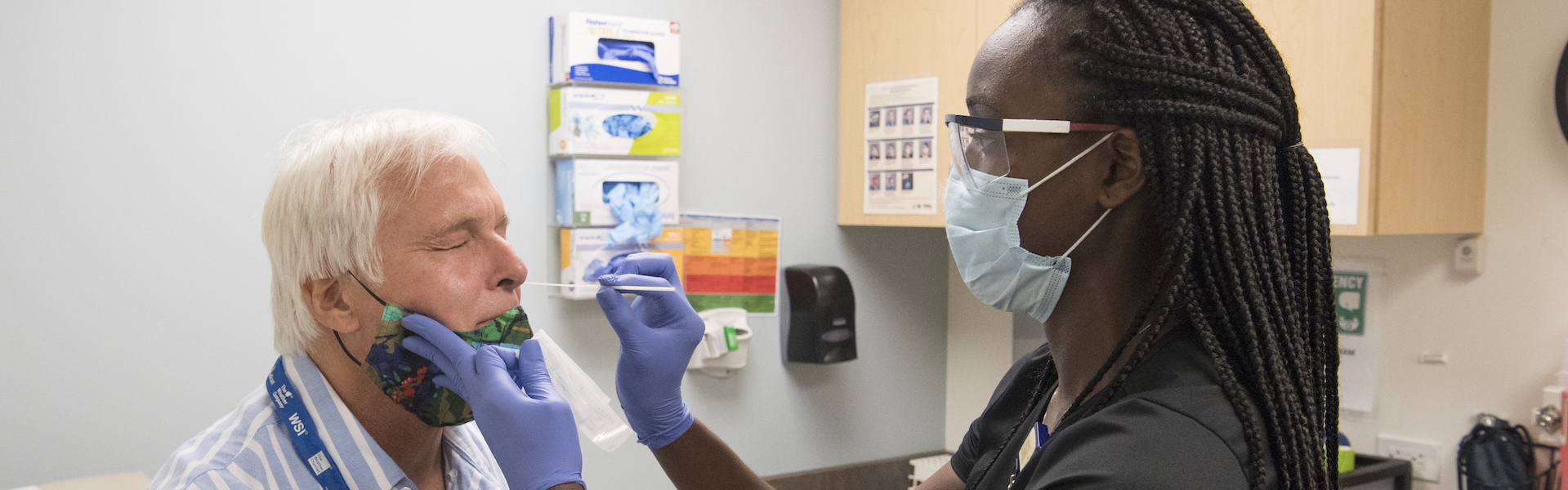
The Infectious Diseases Clinical Research Consortium (IDCRC) and Vaccine and Treatment Evaluation Units (VTEUs) work in tandem with the National Institute of Allergy and Infectious Diseases (NIAID) as a coordinated national and global network of scientific experts working to develop and test vaccines and other therapies to combat infectious diseases.
About
The IDCRC works through a flexible, sustaining structure to advance new countermeasures and approaches to product development, implement efficient clinical study/trial designs, and support exceptional career development and mentoring of future leaders in infectious diseases clinical research. The consortium's collective experience and access to diverse populations give it the ability to evaluate new vaccines and therapies for infectious diseases.

Join us for the 2024 Annual Meeting
Join us on May 1-2, 2024 for our 2024 Annual Meeting. This year's program will highlight the numerous scientific accomplishments of the IDCRC and VTEUs and provide ample networking opportunities for members. We look forward to seeing you soon!
ANNUAL MEETING WEBPAGE
Dr. Kathy Neuzil, named incoming Fogarty International Center Director and NIH Associate Director for International Research
The Infectious Diseases Clinical Research Consortium (IDCRC) Leadership Group and Vaccine & Treatment Evaluation Unit (VTEU) leadership congratulates Kathleen Neuzil, MD, who was announced as the incoming director of the Fogarty International Center and NIH associate director for international research. Dr. Neuzil is the director of the Center for Vaccine Development and Global Health and chief of the Division of Geographic Medicine at the University of Maryland School of Medicine, Baltimore. She also serves as co-principal investigator of the IDCRC Leadership Group (LG). Please join us in congratulating Dr. Neuzil on her new role and expressing our thanks for her exemplary service and contributions to the IDCRC and infectious diseases prevention.
VIEW FULL ANNOUNCEMENT
COVID-19 Prevention Network
The CoVPN was established by merging four existing NIAID-funded clinical trials networks: the HIV Vaccine Trials Network; the HIV Prevention Trials Network; the Infectious Diseases Clinical Research Consortium; and the AIDS Clinical Trials Group.
More about the CoVPN
News from VTEU Trials
IDCRC Leadership Group










IDCRC Concept Quick Stats
ICP Status
- Approved: 56
- Administratively Not Supported: 29
- Not Approved: 51
- EWG Review: 3
- EWG Liaisons: 2
- EMT Concurrence: 0
- Withdrawn: 15
- Hold: 0
- Moved to Active Study: 2
EWG Assignment
- COVID: 92
- Respiratory: 27
- Emerging Infections: 14
- Enteric Infections: 7
- Malaria/Topical Diseases: 13
- STI: 17
ECP
- EWG Review-In Process: 5
- EMT Review: 2
- Approved-moved to Prioritization: 5
- Not Approved: 15
- Approved-moved to Protocol Development: 2
- Active Study: 4
- EMT Vote: 0
- Study in Protocol Development: 6
- Study Closed (LSLV Complete): 6
- Other: 8
IDCRC Citation Statement
Please include the following citation in any publications resulting from direct or indirect IDCRC support:
"Supported by the Infectious Diseases Clinical Research Consortium through the National Institute for Allergy and Infectious Diseases of the National Institutes of Health, under award number UM1AI148684. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health."