Home
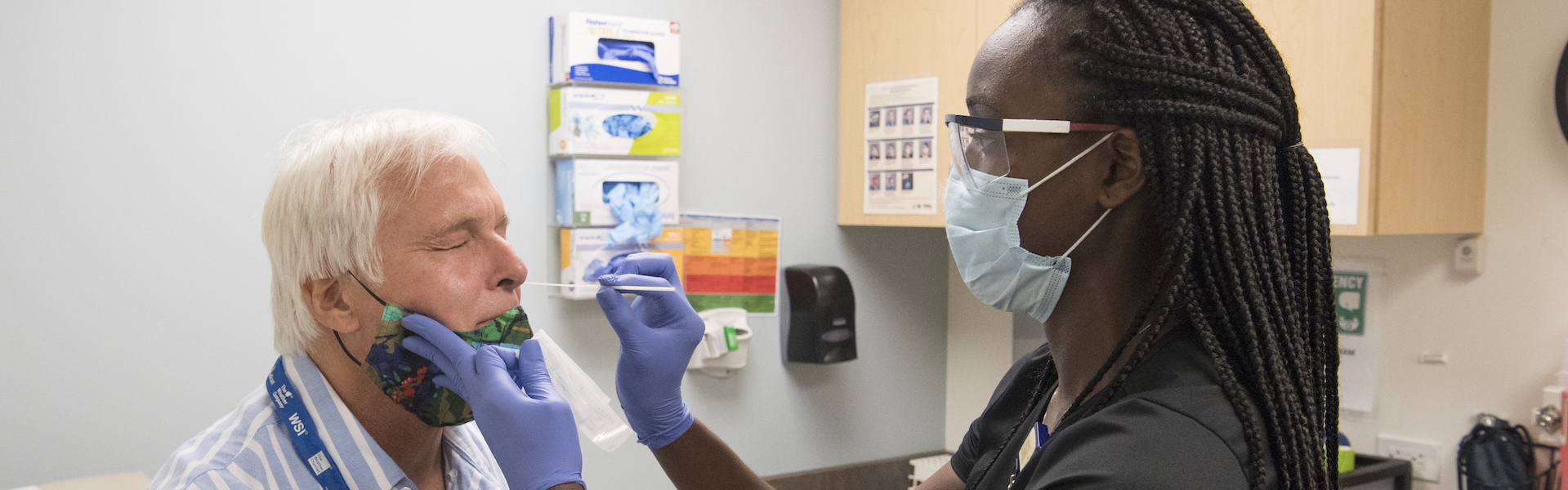
The Infectious Diseases Clinical Research Consortium (IDCRC) and Vaccine and Treatment Evaluation Units (VTEUs) work in tandem with the National Institute of Allergy and Infectious Diseases (NIAID) as a coordinated national and global network of scientific experts working to develop and test vaccines and other therapies to combat infectious diseases.
IDCRC Sites Across the Nation
Legend
- Leadership Group
- VTEU Primary Sites
- VTEU Sub Sites
IDCRC Concept Quick Stats
ICP Status
- Approved: 63
- Administratively Not Supported: 29
- Not Approved: 59
- EWG Review: 0
- EWG Liaisons: 0
- EMT Concurrence: 1
- Withdrawn: 16
- Hold: 1
- Moved to Active Study: 2
EWG Assignment
- COVID: 92
- Respiratory: 34
- Emerging Infections: 15
- Enteric Infections: 8
- Malaria/Topical Diseases: 13
- STI: 18
- Mpox: 7
ECP
- EWG Review-In Process: 0
- EMT Review: 0
- Approved-moved to Prioritization: 3
- Not Approved: 25
- Approved-moved to Protocol Development: 0
- Active Study: 8
- EMT Vote: 1
- Study in Protocol Development: 4
- Study Closed (LSLV Complete): 8
- Other: 10